SoClean 3+ is a Revolutionary Device for Your CPAP Accessory** Care
Leveraging best-in-class engineering and cutting-edge technology, SoClean 3+ reduces bacteria* by 99.9% on CPAP hoses and masks**. This easy-to-use device delivers streamlined performance, meeting FDA standards — and most importantly — your needs.


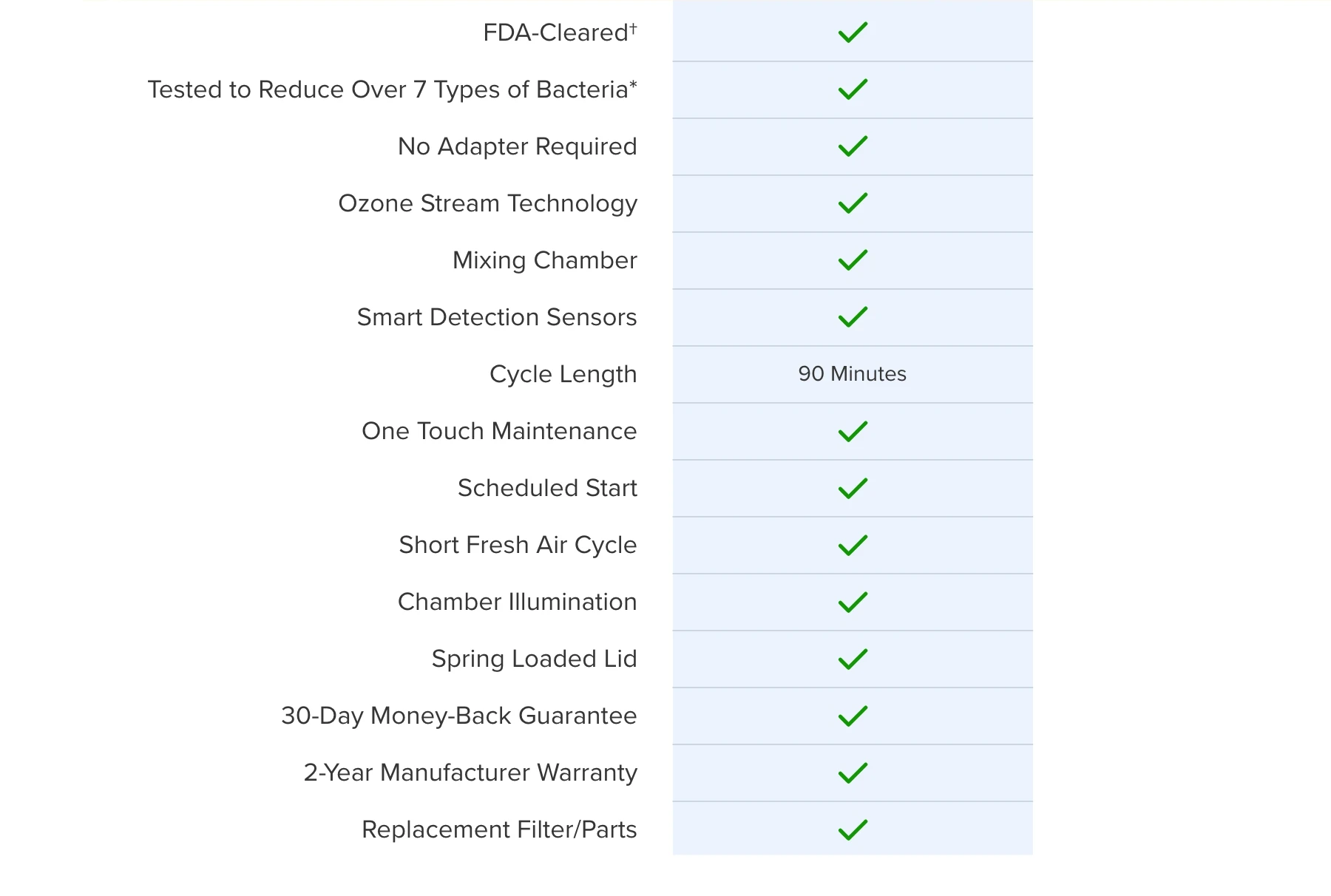
Explore the Advanced Engineering of SoClean 3+
Watch this video for an inside look at the innovation that makes 99.9% bacterial* reduction possible.


Now Available to Reserve. First Come, First Served.
Join the tens of thousands already in line. Reserve your spot commitment-free by entering your email below. You'll receive a notification when SoClean 3+ is available to purchase and a unique $150 coupon.
Your spot is reserved! Stay subscribed to email marketing to ensure you receive your code and can upgrade to the SoClean 3+ as soon as it's available!
Here's How the Program Works




Contraindications For Use
Persons with underlying lung diseases, such as asthma and chronic obstructive pulmonary disease (also known as COPD, which includes emphysema and chronic bronchitis), and those with cardiovascular disease may be sensitive to ozone and should consult with their health care professional before using this product. Safety in pregnant or breastfeeding women and children under the age of 22 have not been established. Consult with your health care professional before using this product.
SoClean 3+ Frequently Asked Questions
The SoClean 3+ is currently established for use with the ResMed Mirage FX (nasal mask), ResMed ClimateLine Air (tubing), and SlimLine tubing for the ResMed AirSense 10.** The safe use of SoClean 3+ with any other respiratory devices or accessories has not been established. For the latest information about SoClean 3+, sign up for updates using the above form.
Hand-washing your CPAP equipment is meant to remove organic material whereas the SoClean 3+ is intended to be used as an adjunct to hand-washing and has been demonstrated to achieve 99.9% bacterial reduction* in CPAP hoses and masks providing you with fresh equipment after each use.
The FDA De Novo clearance is an FDA marketing authorization pathway in which a new type of low to moderate risk medical device classification is established. Obtaining De Novo clearance for the SoClean 3+ means that SoClean worked with the FDA to create a new Class II category named, "Respiratory accessory microbial reduction devices". As the only product in this category, SoClean 3+ is the sole FDA-cleared product that provides users with adjunct bacterial reduction of CPAP hoses and masks.
For more information on the De Novo process, see the FDA's website.
We're excited to bring you the SoClean 3+! The only FDA cleared product in the category. Our team is working hard to bring it to market! We anticipate first units to begin shipping in the next few months. Stay tuned! For the most up-to-date information on the SoClean 3+ product release, sign up for updates using the above form.
No. The SoClean3+ was cleared as an adjunct to your CPAP manufacturer's cleaning instructions.
No, the SoClean 3+ is an over-the-counter device. Once publicly available, it can be purchased online at www.SoClean.com.
Free Shipping: Free standard shipping on most orders over $50. An additional surcharge may apply to some items due to their size and/or weight. See terms and conditions.
30-Day Risk-Free Trial & 2-Year Warranty: Applicable to the SoClean 3, SoClean O3 Smarthome Cleaning System™, and the SoClean Air Purifier+ only. See terms and conditions.
Unlimited Support: Call 866-501-3705 or email info@soclean.com